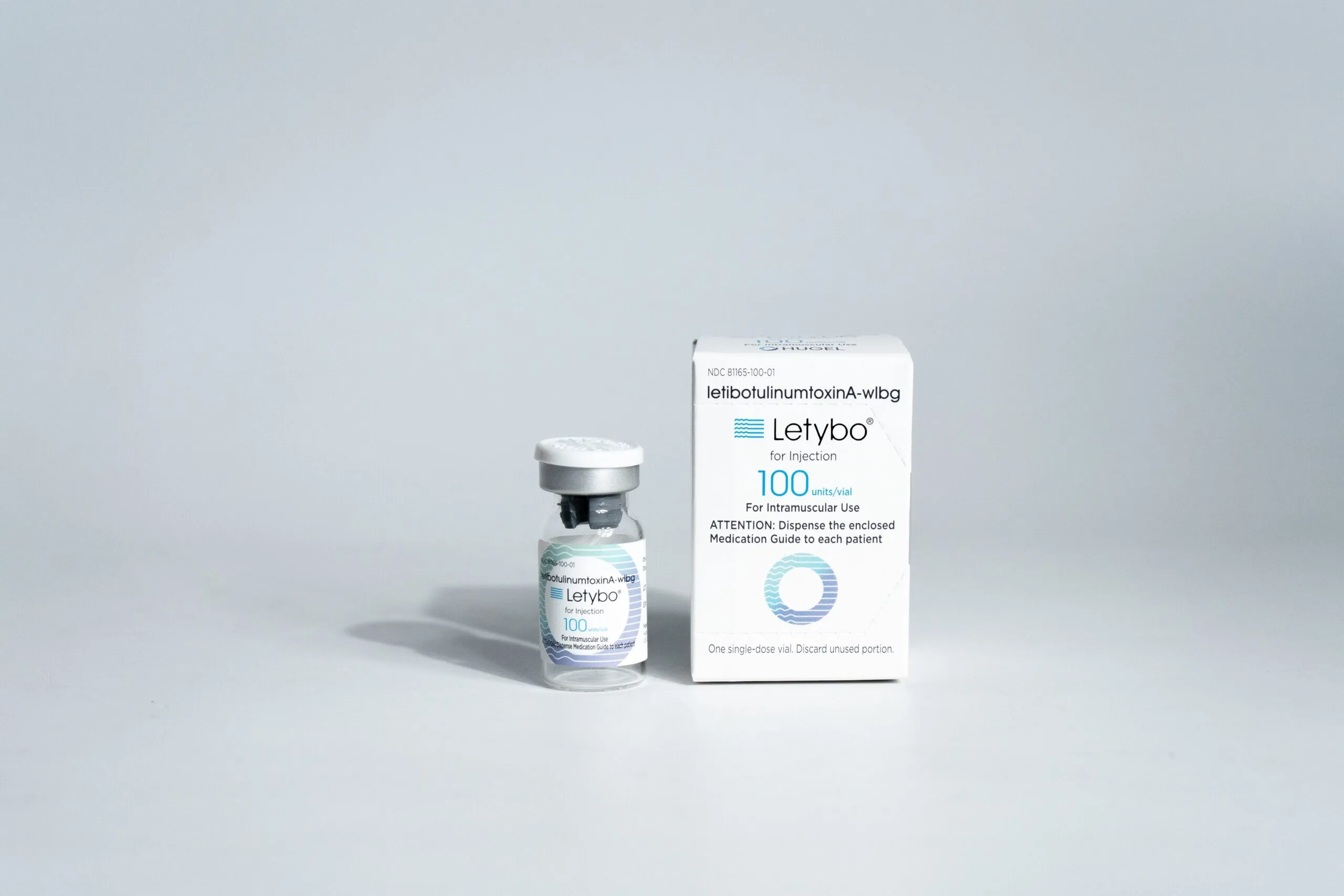
For those seeking a new option in the world of cosmetic injectables, Letybo® (letibotulinumtoxinA-wlbg) is the latest FDA-approved neuromodulator designed to reduce the appearance of moderate to severe glabellar lines (frown lines between the eyebrows). As an alternative to well-known brands like Botox®, Dysport®, and Xeomin®, Letybo® is positioned as a promising treatment for those looking to achieve smoother, younger-looking skin (Allure, 2024).
What is Letybo®?
Letybo® is a botulinum toxin type A injectable treatment that works by temporarily blocking nerve signals to targeted muscles, thereby reducing their movement and softening facial wrinkles (FDA Prescribing Information). It has been widely used in South Korea for years before receiving FDA approval for use in the United States.
How Does Letybo® Compare to Other Neuromodulators?
Like Botox® and other botulinum toxin injectables, Letybo® is used to address fine lines and wrinkles by preventing muscle contractions. However, some preliminary data suggests that Letybo® may have a slightly different diffusion profile, potentially leading to subtle differences in how it spreads under the skin.
One key distinction is that Letybo® is specifically FDA-approved for treating glabellar lines at a dosage of 20 units. While studies are still ongoing, this product is anticipated to be a strong competitor to established brands.
Who Should Avoid Letybo®?
Letybo® is not suitable for individuals who:
- Are allergic to any of its ingredients or any other botulinum toxin product.
- Have an infection at the injection site.
- Have received another botulinum toxin injection within the past four months.
Consultation and Considerations
If you’re considering Letybo®, it’s essential to consult with your provider, who can educate you on safety and potential side effects, as well as how Letybo® compares to other neuromodulators.
Reporting Adverse Reactions
Patients should inform their healthcare providers about any medications, supplements, or recent botulinum toxin treatments before receiving Letybo®. Adverse effects can be reported to Hugel Aesthetics at 1-877-390-2906 or the FDA at 1-800-FDA-1088 (FDA Prescribing Information).

Final Thoughts
Letybo® represents an exciting new option in the world of aesthetic injectables. While it joins a competitive market, its approval offers patients and providers another choice for addressing fine lines and wrinkles. As with any medical aesthetic treatment, it is crucial to seek professional guidance to determine if Letybo® is the right fit for you.
Exclusive Introductory Offer at The Kissed Peach!
Letybo® is now available at The Kissed Peach! For a limited time, the first 10 people to book an appointment will have the opportunity to take advantage of our exclusive intro price of just $10.50 per unit for April bookings only. Don’t miss out on this special offer!